Compartment Syndrome, Lower Extremity
Author: Stephen Wallace, MD, Staff, Department of Emergency Medicine, Eastern Idaho Regional Medical Center
Coauthor(s): Stuart Goodman, MD, PhD, FRCSC, FACS, Associate Chairman, Professor, Department of Functional Restoration, Division of Orthopedic Surgery, Stanford University; Head, Division of Orthopedic Surgery, Stanford University Medical Center; Douglas G Smith, MD, Associate Professor, Department of Orthopedic Surgery, University of Washington, Harborview Medical Center
Contributor Information and Disclosures ( Updated: Feb 9, 2009 )
Introduction
Compartment syndrome (CS) is a condition in which the perfusion pressure falls below the tissue pressure in a closed anatomic space, with subsequent compromise of tissue circulation and function. Each muscle or muscle group is enclosed in a compartment bound by relatively rigid walls of bone and fascia. The compartments of the lower leg and the volar forearm are particularly prone to developing elevated compartment pressures.
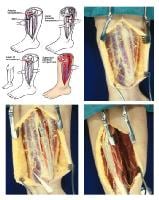
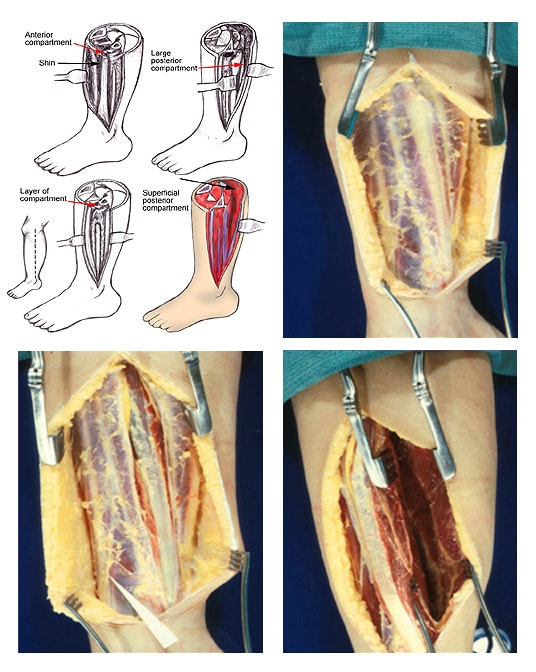
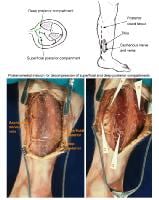
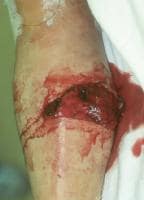
(Click Image to enlarge.) Single-incision fasciotomy. Photographs courtesy of DG Smith, MD, Harborview Hospital, Seattle, WA.
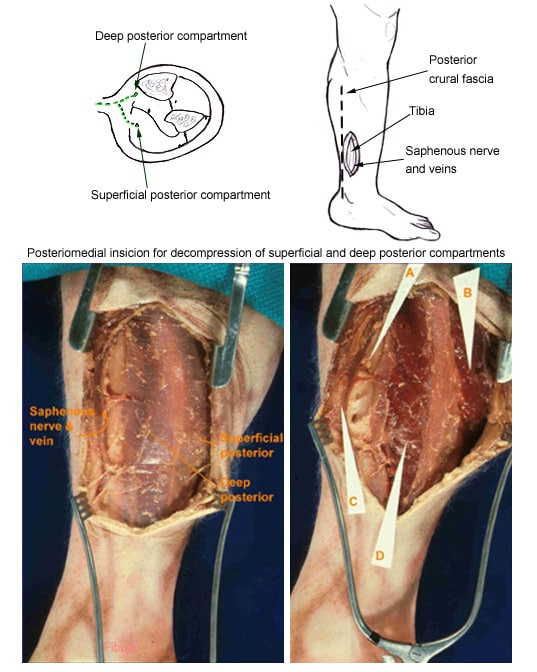
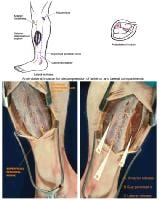
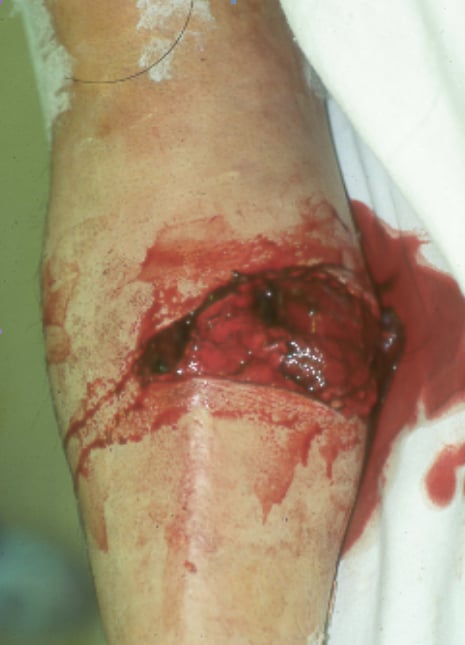
(Click Image to enlarge.) Two-incision posteromedial fasciotomy. Photographs courtesy of DG Smith, MD, Department of Orthopedics, Harborview Hospital, Seattle, WA.
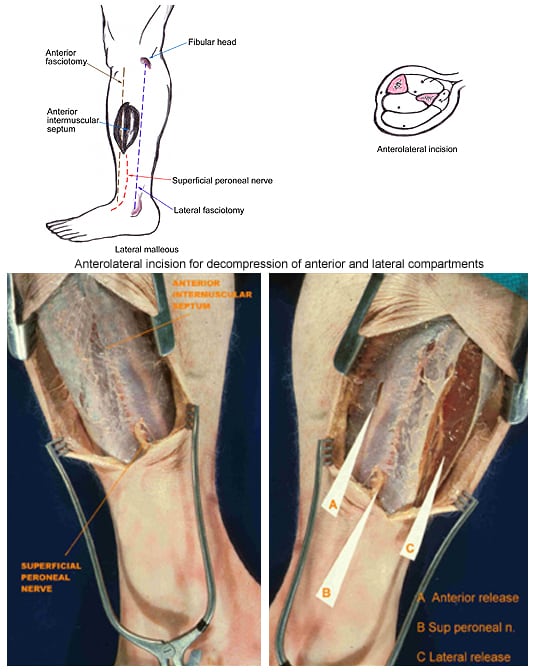
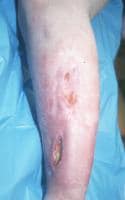
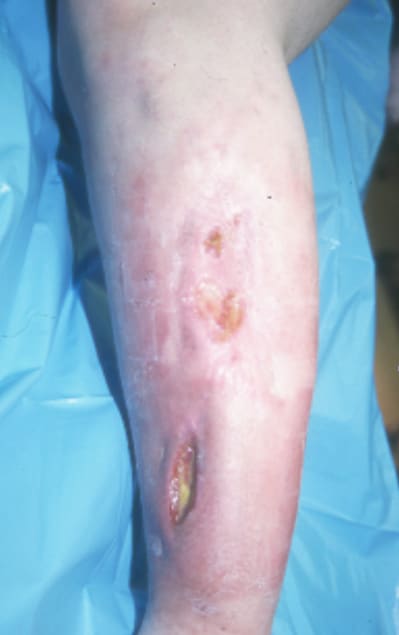
(Click Image to enlarge.) Two-incision anterolateral fasciotomy. Photographs courtesy of DG Smith, MD, Harborview Hospital, Seattle, WA.
As many as 45% of all cases of CS are caused by tibial fractures. Other causes include any long-bone fracture, vascular injury, compression in the setting of a crush injury, drug overdose, and a tight cast or dressing. Late manifestations of CS include the absence of a distal pulse, extremity paresis, and hypoesthesia. If CS is strongly suspected in the clinical examination, operative decompression is the mainstay of therapy. Compartment-pressure measurements are usually reserved for diagnosing chronic CS, for evaluating comatose patients, or for other conditions in which the clinical examination findings are equivocal.
Although rhabdomyolysis and subsequent renal failure are among the most severe life-threatening complications, Volkmann contractures are the more commonly observed limb deformities. This article discusses the current understanding of CS of the lower extremity.
History of the Procedure
The original description of compartment syndrome (CS) was by Richard von Volkmann in 1872.1 Volkmann described contractures of the forearm muscles following tight bandaging for a closed reduction of an elbow fracture. The contractures resulted from ischemic muscle necrosis, which was later termed CS. In 1926, Paul Jepson published his classic paper in which he described prompt surgical decompression for the prevention of paralysis and contracture.2 In 1941, Bywaters and Beall published research on the occurrence of CS following significant crush injuries during World War II.3
In the early 1900s, capsule and balloon measuring devices were implanted surgically and used to make the diagnosis of CS. In 1968, the wick-catheter technique was introduced; its application for compartment-pressure measurements was popularized by Mubarak et al in 1976.4 Almost concurrently in 1976, Matsen et al documented the high incidence of CS and recommended an infusion technique for continuous compartment-pressure measurements.5 More recently, portable surgical-tissue-pressure monitoring devices that are convenient and easy to use have become available from commercial sources.
Chronic CS was not described until 1956 and was thought to be a form of shin splints (anterior tibial enthesitis).6 However, with the advent of the fitness boom and the increased popularity of endurance sports, additional research on exercise-induced leg pain has demonstrated that chronic CS is a well-defined clinical entity.
Problem
Compartment syndrome (CS) occurs whenever increased tissue pressure in a myofascial compartment compromises blood flow to the muscles and nerves within that compartment, resulting in tissue and nerve damage. Two types of CS have been identified, acute and chronic.
Acute CS typically occurs subsequent to a traumatic event, most commonly fractures. Symptoms worsen acutely, and irreversible nerve injury and muscle necrosis occur within hours.
Chronic CS (also known as chronic exertional CS, exertional CS, recurrent CS, or subacute CS) is a recurrent syndrome that occurs with exercise or work. Originally described in 1956, chronic exertional CS was previously thought to be an atypical form of shin splints.6 This syndrome is usually observed in competitive or collegiate athletes. Often, it occurs bilaterally, and similar to claudication, the pain it causes may be reproducible at a specific exercise distance or time interval. For example, most long-distance runners reproducibly experience the onset of pain within 15 minutes of initiating their run. Athletes may not be able to play through the severe pain. However, runners may be able to continue running with a modified flatfoot strike. Symptoms tend to subside within 1 hour of terminating the activity and are minimal during normal daily activities but return when activity is resumed.
Pedowitz et al proposed criteria for the diagnosis of chronic CS.7 One or more of the following is required:
- A resting compartment pressure of greater than or equal to 15 millimeters of mercury (mm Hg)
- A 1-minute postexercise compartment pressure of greater than or equal to 30 mm Hg
- A 5-minute postexercise compartment pressure of greater than or equal to 20 mm Hg (95% confidence level)
Frequency
The incidence of acute compartment syndrome (CS) varies depending on the inciting event. DeLee and Stiehl found that 6% of patients with open tibia fractures developed CS, compared with only 1.2% of patients with closed tibia fractures.8
The reported incidence of CS may underestimate the true incidence because the syndrome may go undetected in severely traumatized patients. The prevalence of CS is much higher in patients who have an associated vascular injury. Feliciano et al reported that 19% of patients with vascular injury required fasciotomy, but other patients have an estimated 30% incidence.9 The true incidence of CS that is associated with vascular trauma may not be known because many vascular surgeons perform a prophylactic fasciotomy at the time of the vascular repair in high-risk patients.
The incidence of chronic CS in athletes has not been determined.
Related eMedicine topics:
Tibia Fractures, Open
Diaphyseal Tibial Fractures
Tibial Plateau Fractures
Tibial Shaft Fractures
The Polytraumatized Patient
Etiology
Compartment syndrome (CS) may be the result of either externally applied compressive forces or internally expanding forces. Fractures, vascular injuries, deep venous thrombosis (DVT), overexertion, fluid sequestration, or prolonged compression (as from a cast or other cause) may lead to CS. As many as 45% of all CS cases are caused by tibial fractures. DVT rarely leads to CS, except in the most severe form of DVT, phlegmasia cerulea dolens.10,11 Acute CS has also been reported to follow minor trauma, such as twisting the calf while running, skiing on moguls, and weightlifting.12 Verdolin et al reported a case of bilateral lower extremity CS following prolonged surgery in the lithotomy position with the use of compression-stocking devices.13
Chronic CS is usually observed in long-distance runners, basketball players, skiers, and soccer players. This condition is usually the result of minor trauma or repetitive microtrauma (overexertion).
The etiology of CS is as follows:
- External restriction of the lower extremity compartment
- Internal increase in compartment volume
- Hemorrhage (trauma, warfarin [Coumadin], tissue plasminogen activator [TPA])
- Hemophilia
- Fractures
- Gunshot wound to thigh
- Massive intravenous (IV) fluid infusion
- Drug/alcohol abuse and coma
- Compartment fluid injection
- Crush injuries
- Rhabdomyolysis
- Gastrocnemius or peroneus muscle tear
- Weightlifting or overuse of weights
- Androgen abuse/muscle hypertrophy
- Knee arthroscopy
- Ruptured Baker cyst
- Snake envenomation
Pathophysiology
Compartment syndrome (CS) develops after elevated compartment pressure causes muscle and nerve ischemia. Tissue perfusion is proportional to the difference between the capillary perfusion pressure (CPP) and the interstitial fluid pressure. This is also stated by the following formula:
LBF = (PA - PV)/R
In the formula above, LBF is local blood flow, PA is local arterial pressure, PV is venous pressure, and R is local vascular resistance.Normal myocyte metabolism requires a 5-7 mm Hg oxygen tension, which can readily be obtained with a CPP of 25 mm Hg and an interstitial tissue pressure of 4-6 mm Hg.16
When fluid is introduced into a fixed-volume compartment, tissue pressure increases and venous pressure rises. When the interstitial pressure exceeds the CPP (a narrowed arteriovenous [AV] perfusion gradient), capillary collapse and muscle and tissue ischemia occur. With myocyte necrosis, myofibrillar proteins decompose into osmotically active particles that attract water from arterial blood. One milliosmole (mOsm) is estimated to exert a pressure of 19.5 mm Hg; therefore, a relatively small increase in osmotically active particles in a closed compartment attracts sufficient fluid to cause a further rise in intramuscular pressure. When tissue blood flow is diminished further, muscle ischemia and subsequent cell edema worsen. This vicious cycle of worsening tissue perfusion continues to propagate. Some reduction in the local AV gradient can be compensated for by changes in local vascular resistance (autoregulation). However, compartment tamponade occurs as arterial blood flow is occluded.
Shrier and Magder questioned this traditional hypothesis for the pathophysiology of CS and postulated that within muscle compartments, a critical closing pressure exists (similar to West zone II in lung physiology).17 The authors showed that the increase in this critical closing pressure, which they called Pcrit, rather than an increase in arterial resistance, results in decreased blood flow.
The transmural pressure at which blood flow ceases depends on adrenergic tone as well as the interstitial pressure; the pressure at which this occurs is still under debate. However, in general, compartmental pressures higher than 30 mm Hg require surgical intervention. Untreated, within 6-10 hours, the final result of such high compartmental pressures is muscle infarction, tissue necrosis, and nerve injury. For unclear reasons, CS that is associated with surgical positioning may manifest later, with a mean time to presentation of 15-24 hours or longer postoperatively.18
Pressure-induced functional deficits are likely due to decreased tissue perfusion rather than a direct mechanical effect. Therefore, the amount of pressure a limb can tolerate depends on limb elevation, blood pressure, hemorrhage, and arterial occlusion. In addition to local morbidity caused by muscle necrosis and tissue ischemia, cellular destruction and alterations in muscle cell membranes lead to the release of myoglobin into the circulation. This circulating myoglobin results in renal injury. Advanced CS may result in rhabdomyolysis, and conversely, rhabdomyolysis may result in CS.19 Patient mortality is usually due to renal failure or sepsis from difficult wound management.
The mechanism of CS following vascular trauma may differ slightly from the above scenario because most cases occur with reperfusion. This reperfusion syndrome is likely related to the ischemic depletion of high-energy phosphate forms and ischemic muscle injury.
The pathogenesis of chronic CS was described by Reneman.20 Muscle bulk increases 20% during exercise and contributes to the transient increase in intracompartmental pressure. Repetitive muscle contraction alone can increase intramuscular pressure to levels that may cause transient ischemia. Chronic CS occurs when the pressure between successive contractions remains high and impedes blood flow. As the pressure rises, arterial flow during muscle relaxation decreases, and the patient experiences muscle cramping. The anterior and lateral compartments of the lower leg are commonly affected; the deep and posterior compartments are less commonly involved.
Presentation
The traditional 5 P's of acute ischemia in a limb (ie, pain, paresthesia, pallor, pulselessness, poikilothermia) are not clinically reliable and manifest only in the late stages of compartment syndrome (CS). Symptomatically, the patient with CS may experience crescendo pain that is out of proportion to the original injury. The pain is also deep and aching in nature and is worsened by passive stretching of the involved muscles. The patient may describe a tense feeling in the extremity. Pain, however, should not be a sine qua non of CS. Paresthesia, or numbness, is an unreliable early symptom.
On physical examination, evidence of trauma and gross deformity should alert the physician to the possibility of a developing CS. Comparison of the affected limb to the unaffected limb is useful. Excessively vigorous examination of a tibial fracture should be avoided because this may exacerbate irritation of the deep posterior compartment.
In the clinical scenario in which there is evidence of trauma and gross deformity, a claw-toe deformity might occur; therefore, the patient should be evaluated for such a condition. Sensory nerves tend to be affected before the motor nerves, and selected nerves may be more susceptible than others in the same compartment. For example, in acute anterior lower leg CS, the first sign to present may be numbness between the first 2 toes (superficial peroneal nerve). Decreased 2-point discrimination is the most consistent early finding, and correlation has also been reported between diminished vibration sense (256 cycles/s) and increasing compartment pressure. On deep palpation, a firm wooden feeling is a specific sign when present. Bullae may also be observed. If objective evidence of a major sensory deficit or loss of peripheral pulse is found, the syndrome is far advanced.
Laboratory testing that reveals a creatine kinase (CK) measurement that is greater than or equal to 1000-5000 U/mL or demonstrates the presence of myoglobinuria may alert the physician to the occurrence of CS.
Patients with chronic CS typically have pain, a tight sensation, and weakness of the muscles of the involved compartment. In anterior CS, a runner may develop foot slap on heel-strike due to weakness of the tibialis anterior.
Indications
In the setting of classic compartment syndrome (CS) presentation and physical examination findings, no further diagnostic workup is needed. No consensus exists regarding the pressure for which fasciotomy should be performed. Whitesides et al noted that fasciotomy should be performed when compartment pressure rises to within 10-30 mm Hg of the patient's diastolic blood pressure (this value has been coined the delta-P).6 McQueen and Court-Brown, studying CS in dogs, affirmed the difference of 30 mm Hg between the compartment pressure and the diastolic blood pressure as a more reliable measure than absolute pressure measurements.21 Many surgeons now use a measured compartment pressure of 30 mm Hg as a cutoff for fasciotomy. Multiple pressure readings are often obtained, and the clinician must decide how to incorporate these readings with the clinical picture in the decision-making process.
In addition to direct pressure measurements, other less invasive compartment blood flow–measurement techniques have been studied. These include laser Doppler, methoxy isobutyl isonitrile–enhanced magnetic resonance imaging (MIBI MRI), phosphate nuclear MRI (P-NMR), thallium-201 (201 Tl) and technetium-99m (99m TC) sestamibi, and xenon (Xe) scanning.
In the setting of a vascular injury, a fasciotomy should be performed on high-risk patients before arterial exploration. High-risk patients include those with prolonged ischemia time, significant preoperative hypotension, associated crush injury, combined arterial and venous injury, or the need for a major venous ligation in the popliteal or femoral area.
Relevant Anatomy
- Anterior compartment
- Dorsiflexion muscles of the ankle and foot
- Tibialis anterior
- Extensor digitorum longus
- Extensor hallucis longus
- Peroneus tertius
- Anterior tibial artery – Commonly injured in lateral tibial plateau fractures
- Deep peroneal nerve – Provides sensation to the first dorsal web space
- Dorsiflexion muscles of the ankle and foot
- Lateral compartment
- Peroneus brevis and peroneus longus – Plantar flexor and evertor muscles of the foot
- Superficial peroneal nerve – Provides sensation to the dorsum of the foot
- Deep posterior compartment
- Plantar flexor and phalangeal flexor muscles
- Tibialis posterior
- Flexor digitorum longus (FDL)
- Flexor hallucis longus
- Posterior tibial and peroneal arteries
- Posterior tibial nerve – Provides sensation to the sole of the foot
- Plantar flexor and phalangeal flexor muscles
- Superficial posterior compartment
- Plantar flexor muscles of the foot
- Gastrocnemius
- Plantaris
- Soleus
- Sural nerve – Provides sensation to the lateral aspect of the foot and distal calf
- Plantar flexor muscles of the foot
Contraindications
If compartment syndrome (CS) is diagnosed late, fasciotomy is of little benefit. In fact, fasciotomy is probably contraindicated after the third or fourth day following the onset of CS, and when performed late, severe infection usually develops in the necrotic muscle.
______________________________________________________________________________________
Tibial Shaft Fractures
Author: Brian K Konowalchuk, MD, Staff Physician, Department of Orthopedic Surgery, University of Minnesota College of Medicine
Coauthor(s): Nicholas J Wills, MD, Fellow, Twin Cities Spine Center; Brian Tollefson, MD, Flight Surgeon, United States Air Force; Marc Swiontkowski, MD, Chair, Professor, Department of Orthopedic Surgery, University of Minnesota at Minneapolis
Contributor Information and Disclosures ( Updated: May 15, 2009 )
Introduction
An understanding of the diagnosis and treatment of tibial shaft fractures is of importance to primary care physicians and orthopedic surgeons alike. Often, the primary care provider first comes into contact with tibial shaft fractures and must make the diagnosis and early treatment decisions. High-speed lifestyles with motor vehicles, snowmobiles, and motorcycles, as well as the growing popularity of extreme sports, contribute to the increasing occurrence of tibial shaft fractures in today's society. In fact, the tibia is currently the most commonly fractured long bone in the body.
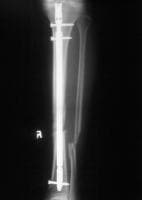
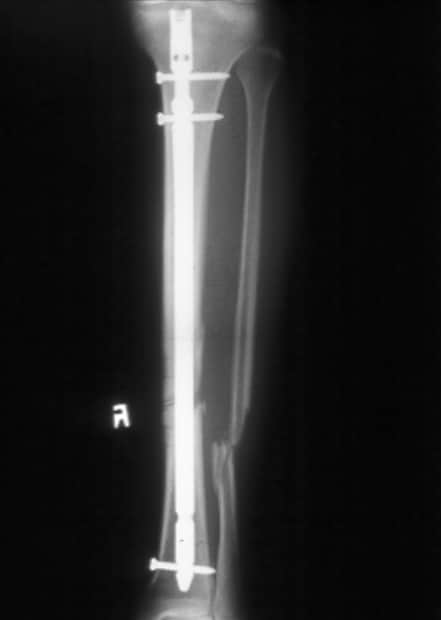
Standard anteroposterior radiograph of a tibial shaft fracture with intramedullary nail fixation. Note the commonly associated fibular fracture that is also apparent.
For excellent patient education resources, visit eMedicine's Breaks, Fractures, and Dislocations Center. Also, see eMedicine's patient education articles Broken Leg, Ankle Fracture, and Knee Dislocation.
History of the Procedure
Plating tibial shaft fractures was the treatment of choice 2-3 decades ago. Intramedullary nailing and external fixation have replaced fracture plating because they are associated with decreased technical difficulty, lower infection rates, and less damage to local soft tissues (see Treatment, Intraoperative details, below).
Problem
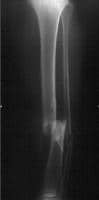
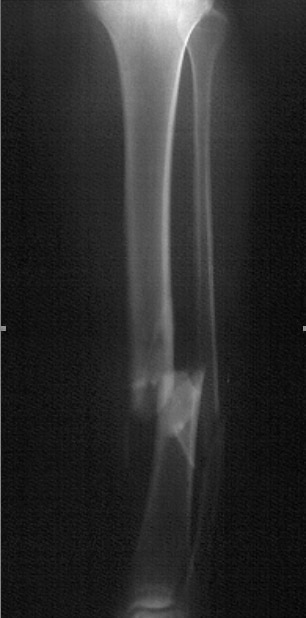
The tibia is the most commonly fractured long bone in the body. Tibial shaft fractures are often the result of high-speed trauma but can also be insidious in onset, such as stress fractures in active individuals. During the initial evaluation, the patient with a tibial shaft fracture should be evaluated carefully for open wounds at the fracture site, neurovascular sufficiency, and elevated compartment pressures. Abnormalities in any of these areas constitute a surgical emergency.
Frequency
The tibia is currently the most commonly fractured long bone in the body. Alho et al have reported an annual incidence of 2 tibial shaft fractures per 1000 individuals.1 The average age of patients with tibial shaft fractures is approximately 37 years, and teenage males are reported to have the highest incidence.2
Etiology
In the etiology of tibial fractures, high-speed trauma is paramount. In areas where people drive cars at high speeds and engage in activities with high potential for leg trauma (eg, skiing, soccer), the number of tibial fractures seen in the emergency department is high. While a direct blow to the tibia is the most common cause, countless other etiologies for tibial shaft fractures are encountered. Two of the most prevalent are falls or jumps from significant height and gunshot wounds to the lower leg.
Presentation
Patients with tibial shaft fractures report pain of varying degrees, but pain is usually severe. An inability to bear weight on the affected leg and a visible malformation of the leg are often present. Partial fractures may be less characteristic in presentation. The evaluating physician should always keep tibial fracture as part of the differential diagnosis after trauma, especially in a patient with an altered mental status who cannot provide a reliable history.
If the patient's symptoms stem from a stress fracture, the patient may reveal a recent change in lifestyle or an increase in physical activity. The pain is worse with weightbearing exercise and improves with rest. A classic presentation is an athlete who did not participate in conditioning work during summer vacation and presents to the physician's office 2 weeks after beginning vigorous training in a fall sport.3
Whatever the presentation, taking a complete history and performing a thorough physical examination are important. The history should include the patient's description of the events that brought him or her to the office. Important details to obtain from the patient include exactly what the patient was doing at the time of the injury, the amount of time that has passed since the injury occurred, a description of pain, any associated paresthesias or numbness, and a history of previous conditions that predispose to this injury or complicate surgery.
During the physical examination, the physician should not focus just on the leg, because concomitant injury is common with tibial fractures. After the other aspects of the examination have been addressed, the physician should specifically attempt to assess the neurovascular status of the patient's injured leg. The results of these examinations are important because their outcomes determine the emergent level of the situation and dictate which surgical specialists must be consulted. The overlying skin should also be examined, with particular care taken in assessing any open wounds or color changes that may indicate a more serious injury.
Classification and nomenclature
Classifications for fractures are useful for consistent communication between physicians. They have been used to predict probability of fracture union and, hence, as a guide for fracture treatment.4,5,6,7,8,9 The classic classification for open fractures was described by Gustilo et al, as follows10 :
- Type I: The wound is clean and is shorter than 1 cm.
- Type II: The wound is longer than 1 cm and does not have extensive soft tissue damage.
- Type IIIa: This fracture type is a wound associated with extensive soft tissue damage usually larger than 10 cm with periosteal coverage. (Periosteum is the outermost layer of bone. It has a rich vascular supply and is important in bone growth and repair.) This fracture type also includes less traumatic fractures with increased chances of complications, such as gunshot wounds, farmyard injuries, and fractures requiring vascular repair.
- Type IIIb: This type is defined as bone with periosteal stripping that must be covered; these fractures nearly always require flap coverage.
- Type IIIc: This type of injury requires vascular repair.
The Orthopaedic Trauma Association has also adopted a system of classification for tibial shaft fracture. Their system, based on radiographic evaluation, divides fractures into 3 main categories (ie, A, B, and C).11,12 Each category is divided into 3 groups, and each group is then further divided into 3 subgroups.
- Type A: This type encompasses unifocal fractures. The subgroups (A1, A2, and A3) are determined by the angle of the fracture. Spiral fractures make up group A1, oblique fractures are A2, and transverse fractures are classified as A3.
- Type B: These are wedge fractures. This category is then further divided into intact spiral wedge fractures, intact bending wedge fractures, and comminuted wedge fractures. These are classified as groups B1, B2, and B3, respectively. The 3 subgroups for both type A and type B fractures are determined by the extent of fibular injury and location of fracture with respect to the tibia.
- Type C: Complex fractures are classified as type C. C1 fractures are spiral wedge fractures. The number of fragments present determines C1 subgroups. C1 subgroups are assigned according to the number of fracture fragments present, with 2 or 3 fragments being classified as C1.1 or C1.2, respectively. Fractures with more than 3 fragments are classified as C1.3 in this system. Segmental fractures are type C2 fractures. C2 fractures, like C1 fractures, are subclassified by the number of fracture fragments present. Highly comminuted fractures are labeled as C2.3. The subdivisions in this group are classified according to the number of fracture fragments and the extent of comminution.
Indications
Most closed tibial fractures can be treated nonoperatively with good result, but infection risk and fracture stability must be considered. Littenberg et al reviewed 2372 case reports of closed tibial fractures and compared clinical outcomes of cast treatment, open reduction and internal fixation, and intramedullary rod therapy. They showed cast treatment to be associated with fewer superficial infections than open reduction and internal fixation. Open reduction and internal fixation, however, demonstrated a higher union rate at 20 weeks.13
In some instances, the fracture cannot be treated properly with nonoperative methods. Operative fixation is required when fractures are unstable. Instability is defined as greater than 1.5 cm of shortening, more than 5° of varus or valgus angulation, 10° of anterior or posterior angulation, and/or less than 50% translation while the leg is in a cast. Factors that contribute to instability are the degree of comminution, the presence of ipsilateral fibular fractures, and the location of the fracture along the tibia. The original presenting radiograph is useful because, often with cast or brace treatment, the original amount of shortening is what the fracture ultimately heals with; therefore, shortening greater than 1 cm is a relative indication for operative stabilization.
Open fractures are surgical emergencies, and an orthopedic surgeon should be consulted immediately. Rarely, a type I fracture can be treated nonoperatively, but most patients should be scheduled for debridement and irrigation within 6 hours of the injury. Longer intervals have been shown to increase rate of infection.14 Patients with Gustilo type II and III open fractures should always be taken to the operating room for irrigation, debridement, and possible surgical fixation (eg, intramedullary nailing, external fixation, plating). Situations in which an open fracture should not be corrected emergently are rare. In some cases, however, especially in the polytrauma scenario, definitive fracture treatment may be delayed. If surgery must be delayed, leg appearance and compartmental pressure must be monitored carefully.
Relevant Anatomy
The leg is divided into 4 distinct fascial compartments. The compartmental anatomy can become extremely important during a traumatic situation in which internal bleeding in the leg can lead to a compartment syndrome.
The anterior compartment contains the dorsiflexors of the foot, including the tibialis anterior, extensor digitorum longus, extensor hallucis, and peroneus tertius. Also housed in the anterior compartment is the deep peroneal nerve. The major blood supply to the anterior compartment is from the anterior tibial artery and its associated vessels.
The lateral compartment contains the peroneus longus and brevis muscles, which primarily serve in eversion of the foot. The superficial peroneal nerve is contained in this compartment and innervates these 2 muscles.
The posterior aspect of the leg is divided into 2 compartments, superficial and deep. The deep compartment contains the plantarflexor muscles, including the tibialis posterior, flexor hallucis longus, and flexor digitorum longus. The peroneal and the posterior tibial arteries also course through this compartment with their corresponding veins. The superficial posterior compartment is the largest of the 4 compartments but contains only muscle. These plantarflexing muscles include the soleus, the gastrocnemius, and the plantaris.
Contraindications
Several contraindications to surgery are recognized for the treatment of tibial shaft fractures. All patients require a thorough preoperative evaluation and must be cleared for general anesthesia prior to any operation, including treatment of tibial shaft fractures. In cases of acute trauma, patients should be stabilized by the trauma team prior to fixation of a tibial shaft fracture. Incision and drainage of infected fracture sites often is indicated; however, hardware should never be placed into an infected wound. In cases in which infected hardware is removed, treat the infection with intravenous antibiotics and replace the hardware in a second surgery after the infection has been treated thoroughly.
More on Tibial Shaft Fractures |
 Overview: Tibial Shaft Fractures Overview: Tibial Shaft Fractures |
| Workup: Tibial Shaft Fractures |
| Treatment: Tibial Shaft Fractures |
| Follow-up: Tibial Shaft Fractures |
| Multimedia: Tibial Shaft Fractures |
_____________________________________________________________________
Introduction
Traumatic injuries of the scapula have received little attention in the literature because they are uncommon. Scapula fractures account for approximately 1% of all fractures. Most scapula fractures can be managed effectively with closed treatment. Some injuries with significant displacement have poor long-term outcomes for the shoulder and the upper extremity as a whole if treated with closed techniques. This article reviews closed management of scapula fractures, discusses open treatment, and provides guidelines for injuries that require operative intervention.
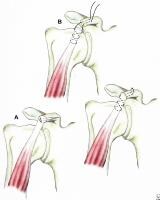
Fixation of acromion fractures. (A) tension band construct; and (B) plate-screw fixation (most appropriate for proximal fractures).
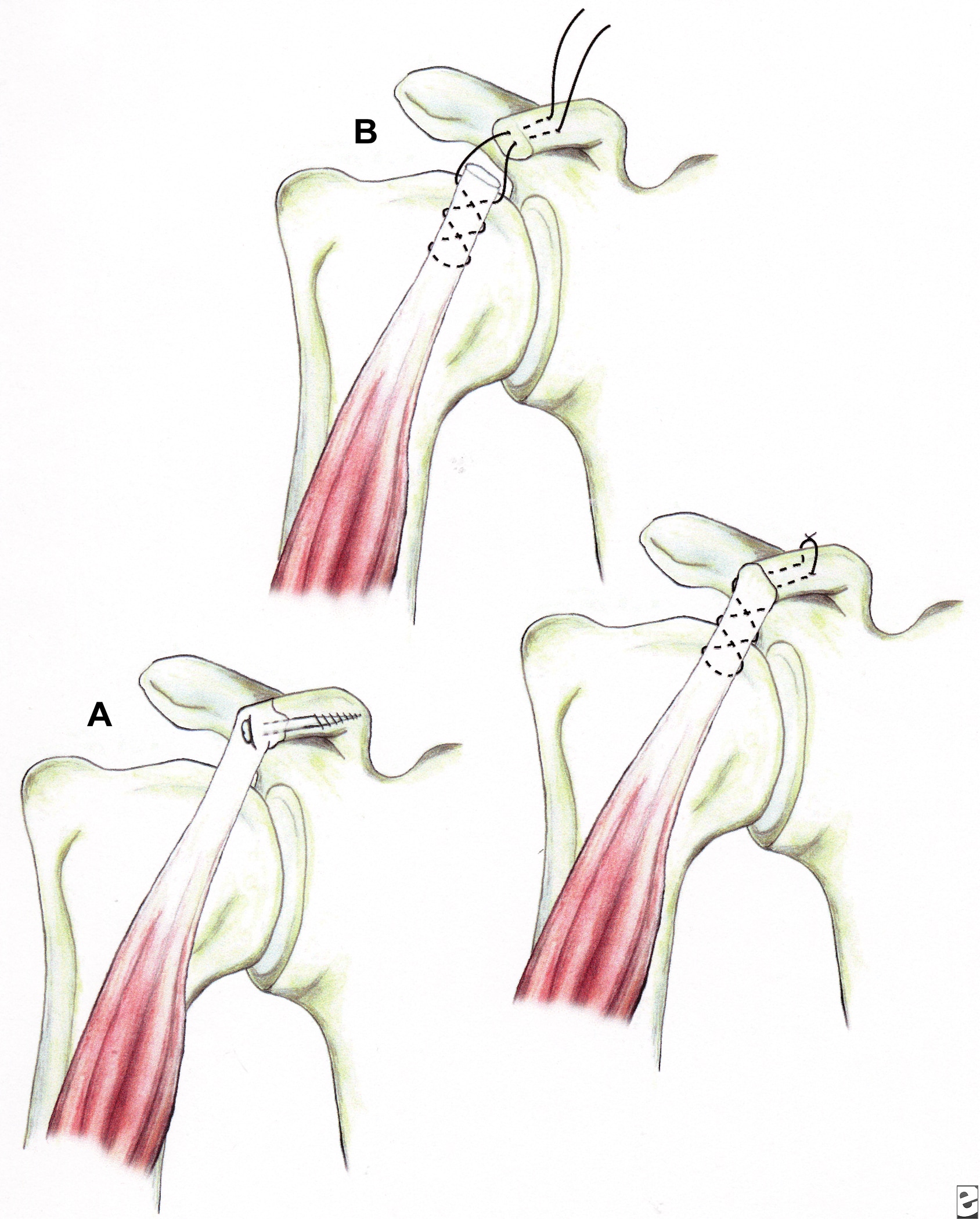
Illustrations showing techniques for managing coracoid fractures. (A) interfragmentary screw fixation (if the fragment is sufficiently large and noncomminuted), and (B) excision of the distal fragment (if small and/or comminuted) and suture fixation of the conjoined tendon to the remaining coracoid process.
History of the Procedure
Historically, scapula fractures have been treated by closed means. One of the earliest descriptions of treating scapula fractures was published in 1805 in Desault's treatise on fractures.
Hardegger et al reported that if significant displacement occurs, conservative treatment alone cannot restore congruence, and stiffness and pain may result, thereby indicating open reduction and stabilization.1
Problem
As previously mentioned, scapula fractures are relatively uncommon. However, they do have a high association with other injuries.2,3 Research shows that 80-95% of scapula fractures have associated injuries. The associated injuries may be multiple and/or life-threatening. As a result, diagnosis and treatment of scapular injuries may be delayed or suboptimal. Long-term functional impairment may occur. As more focus is placed on the proper management of scapular injuries, functional outcomes should improve.
Associated injury patterns commonly involve the ipsilateral upper extremity and thorax.
- Frequencies of associated injuries are as follows:
- Rib fractures - 25-45%
- Pulmonary injury (eg, hemopneumothorax, pulmonary contusion) - 15-55%
- Humeral fractures - 12% (5-10% sustain a brachial plexus injury)
- Skull fractures - 25%
- Central neurologic deficits 5%
- Major vascular injury - 11%
- Splenic injury requiring splenectomy - 8%
Related eMedicine topics:
Proximal Humerus Fractures
Brachial Plexus Injuries, Traumatic
Skull Fracture
Closed Head Trauma
Frequency
Scapula fractures account for 1% of all fractures, 3% of shoulder girdle injuries, and 5% of all shoulder fractures. Approximately 50% of scapula fractures involve the body and spine. Fractures of the glenoid neck constitute about 25% of all scapula fractures, whereas fractures of the glenoid cavity (glenoid rim and fossa) make up approximately 10% of scapula fractures. The acromial and coracoid processes account for 8% and 7%, respectively.
Etiology
Typically, scapula fractures result from high-energy trauma. Direct forces are most common, but indirect mechanisms can also be responsible. An example of an indirect force is a fall on an outstretched arm that causes the humeral head to impact on the glenoid cavity.
Presentation
Most patients with scapula fractures present after high-energy trauma. Associated injuries are common and may delay the diagnosis. Typically, physical examination reveals swelling, tenderness, crepitus, and ecchymosis over the scapular region. Perform a careful neurovascular examination to rule out arterial injury or brachial plexopathy.
Indications
While most scapula fractures can be managed with closed treatment, consider surgical management for significantly displaced fractures.4,5 The following injuries occur with enough frequency to merit discussion of operative treatment:
- Significantly displaced fractures of the glenoid cavity (glenoid rim and fossa)
- Significantly displaced fractures of the glenoid neck
- Double disruptions of the superior shoulder suspensory complex (SSSC) in which 1 or more elements of the scapula are significantly displaced
Approximately 50% of scapula fractures involve the scapular body and spine. Avulsion fractures caused by indirect forces and injuries caused by direct trauma have been described. The latter may be severely comminuted and displaced. Despite sporadic reports describing operative management, there seems to be little enthusiasm for surgical treatment. The 2 reasons for this reluctance are that (1) there is little substantial bone stock for internal fixation, aside from the scapular spine and lateral scapular border; and (2) these fractures seem to heal reliably with a good functional result without surgical treatment. If painful scapulothoracic impingement occurs at a later date, bone prominences over the ventral scapular surface can be removed surgically.
Significantly displaced fractures of the glenoid cavity (rim and fossa)
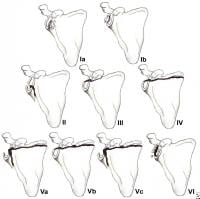
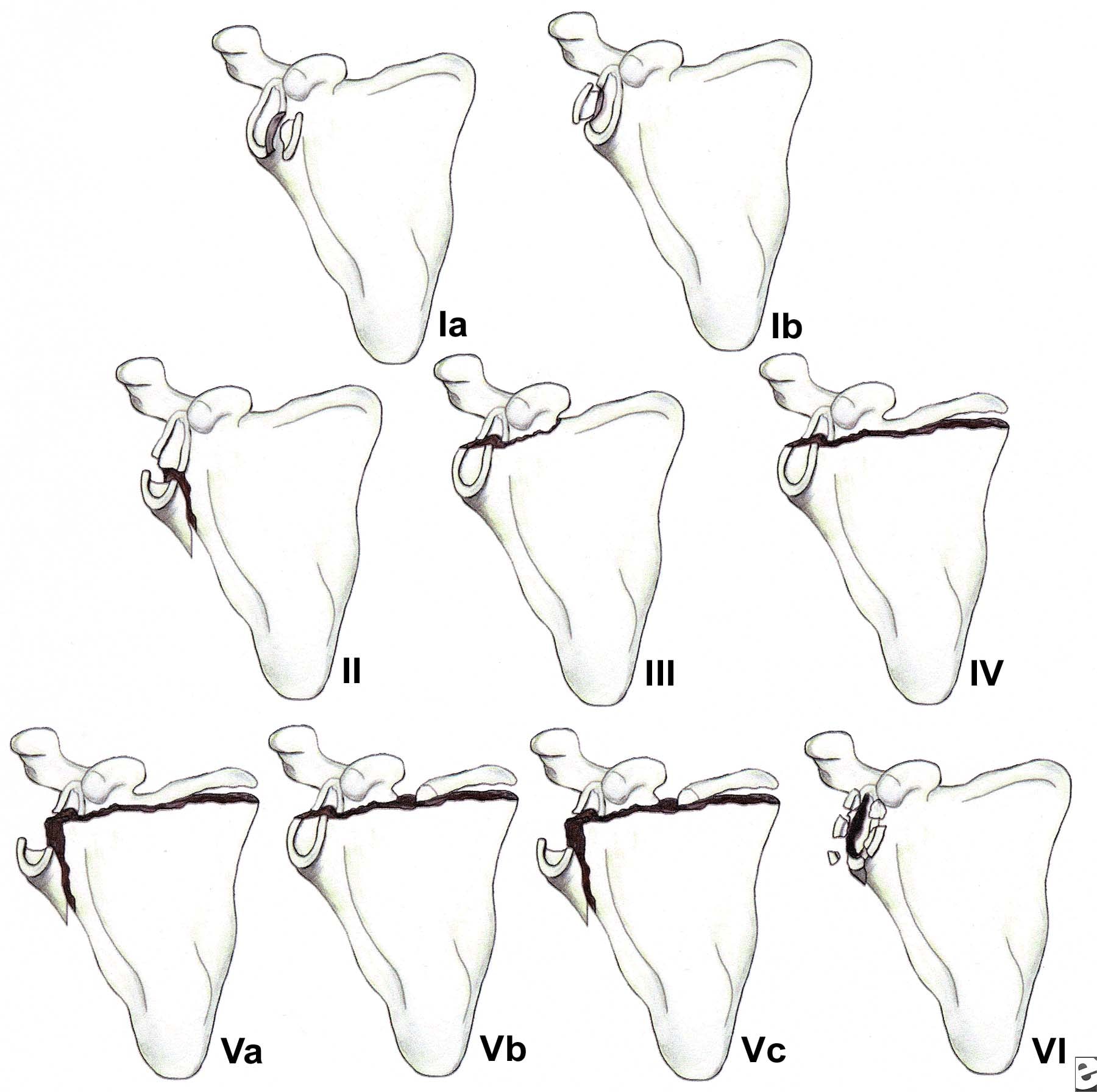
Less than 10% of glenoid cavity fractures are significantly displaced. Ideberg reviewed over 300 such injuries and proposed the first detailed classification scheme.6 This classification subsequently was expanded by Goss (see Image below and Image 1 in Multimedia).7 Type I injuries involve the glenoid rim (IA = anterior rim, IB = posterior rim). Types II-V include fractures of the glenoid fossa. Type VI fractures include all comminuted injuries (ie, more than 2 glenoid cavity fragments).
(Click Image to enlarge.) Classification of glenoid cavity fractures: IA - Anterior rim fracture; IB - Posterior rim fracture; II - Fracture line through the glenoid fossa exiting at the lateral border of the scapula; III - Fracture line through the glenoid fossa exiting at the superior border of the scapula; IV - Fracture line through the glenoid fossa exiting at the medial border of the scapula; VA - Combination of types II and IV; VB - Combination of types III and IV; VC - Combination of types II, III, and IV; VI - Comminuted fracture
Fractures of the glenoid rim occur when the humeral head is driven against the glenoid margin. Surgical management is indicated if the fracture results in persistent subluxation of the humeral head, defined as failure of the humeral head to lie concentrically within the glenoid fossa, or if the reduction is unstable. DePalma has stated that instability can be anticipated if the fracture is displaced 10 mm or more and if at least one fourth of the anterior aspect of the glenoid cavity or one third of the posterior aspect of the glenoid cavity is involved.8
Fractures of the glenoid fossa occur when a laterally applied force drives the humeral head directly into the glenoid cavity. Soslowsky et al found the maximum depth of glenoid articular cartilage to measure 5 mm.9 Consequently, with displacement more than 5 mm, subchondral bone is exposed, making posttraumatic arthritis more likely. Kavanagh et al reported on open reduction and internal fixation (ORIF) of glenoid fossa fractures in which displacement ranged from 4 to 8 mm.10 They found ORIF to be a useful and safe technique for the treatment of selected displaced fractures of the glenoid fossa. Based on these and other studies, it seems reasonable to conclude that an articular step-off of 5 mm should warrant consideration for ORIF, and a 10-mm step-off is a definite indication for surgery.
Other indications for surgical management include (1) glenoid fossa fractures that result in significant displacement of the humeral head such that it fails to lie in the center of the glenoid cavity, thereby resulting in glenohumeral instability; and (2) fractures of the glenoid fossa with such severe separation of the fracture fragments that nonunion is likely to occur.
Glenoid neck fractures
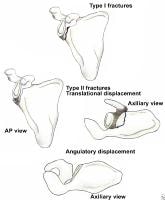
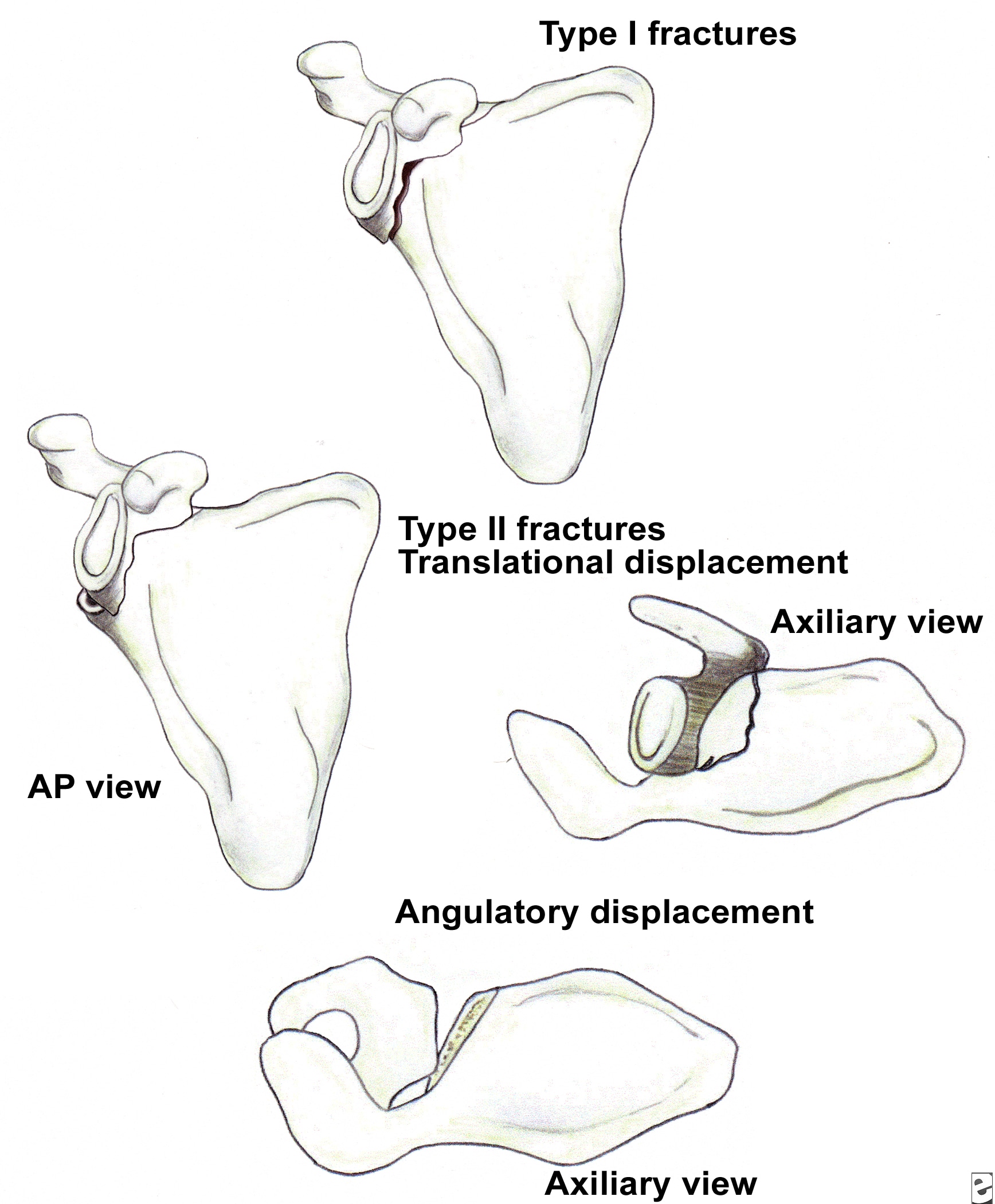
Glenoid neck fractures that cause significant translational or angulatory displacement of the glenoid fragment can interfere with normal shoulder mechanics and/or cause glenohumeral instability. Nordqvist and Petersson evaluated 37 glenoid neck fractures treated nonoperatively and found the functional results at 10- to 20-year follow-up to be fair or poor in 32% of cases.11 Hardegger et al noted that displaced glenoid neck fractures result in a functional imbalance because the relationship of the glenohumeral joint with the acromion and nearby muscle origins is altered.1
Classification of glenoid neck fractures. Type I includes all minimally displaced fractures. Type II includes all significantly displaced fractures (translational displacement greater than or equal to 1 cm; angulatory displacement greater than or equal to 40°)
Overall, there is literature to suggest that surgery should be considered for fractures with translational displacement greater than or equal to 1 cm and/or angulatory displacement greater than or equal to 40° in either the transverse or coronal plane (see Image above and Image 2 in Multimedia).
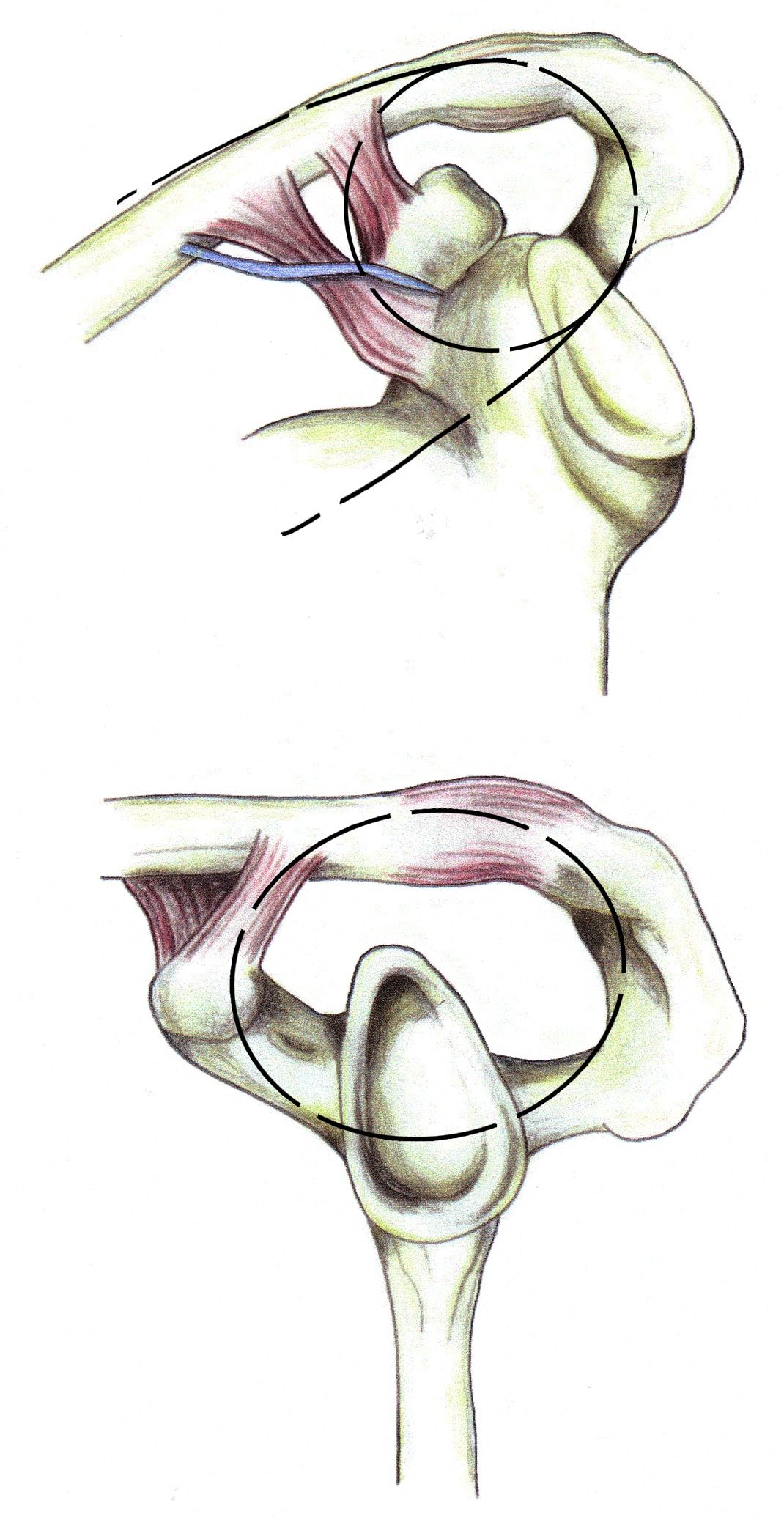
Double disruptions of the SSSC
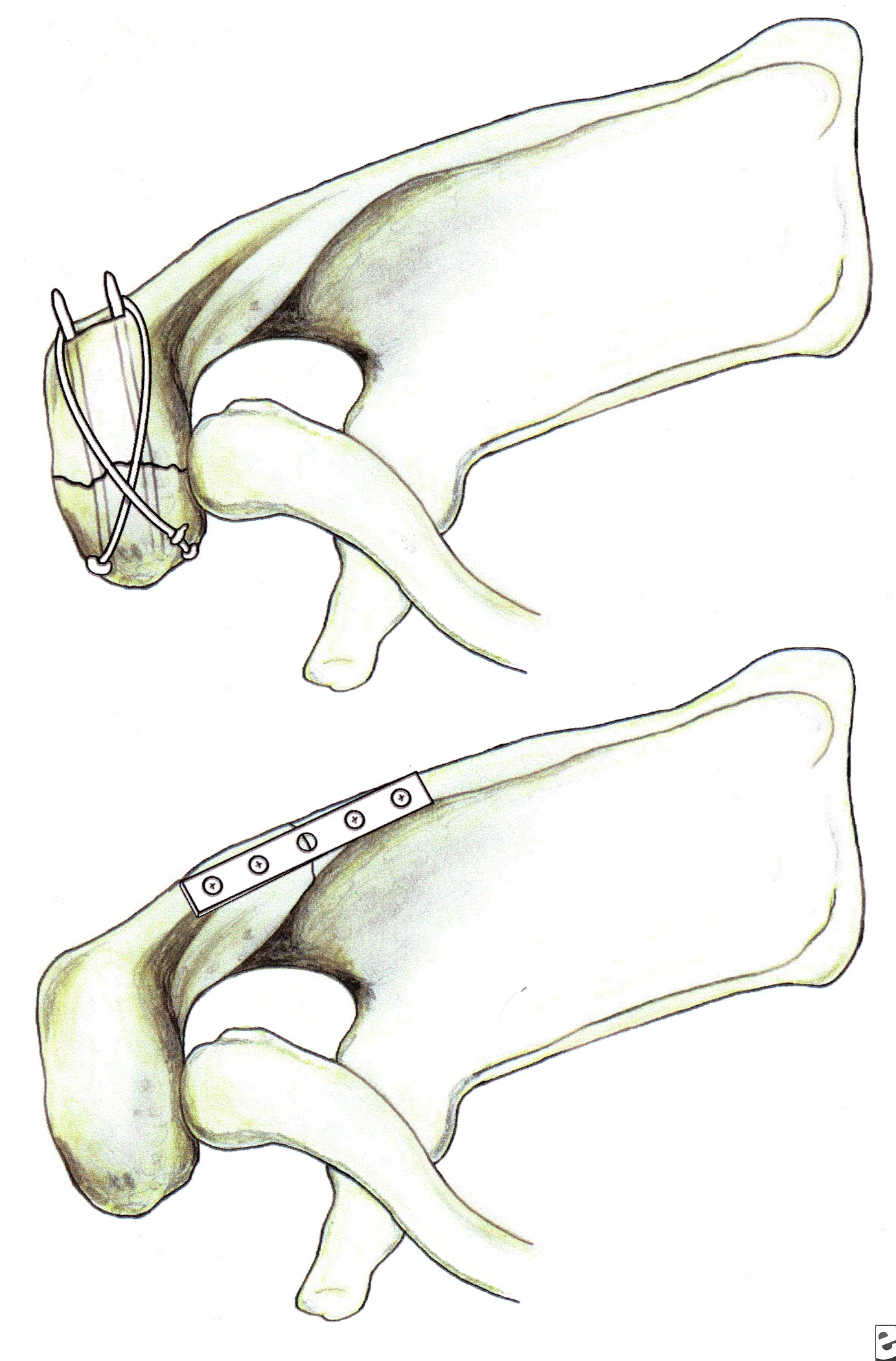
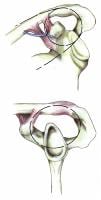
The SSSC is a bone/soft-tissue ring at the end of a superior and inferior bony strut (see Image below and Image 3 in Multimedia). The ring consists of the glenoid process, coracoid process, coracoclavicular ligaments, distal clavicle, acromioclavicular (AC) joint, and acromial process. The superior strut is the middle third of the clavicle. The inferior strut is the lateral scapular body and spine.
Superior shoulder suspensory complex. (A) anteroposterior view of the bony/soft tissue ring and the superior and inferior bony struts; and (B) lateral view of the bony/soft tissue ring.
Traumatic disruptions of 1 of the components of the SSSC are common. If the force is sufficient, the ring may fail in 2 or more places (double disruption), a situation in which significant displacement at 1 or both of the individual sites and of the SSSC as a whole frequently occurs. Similarly, a disruption of 1 portion of the ring, combined with a fracture of 1 of the struts or fractures of both struts, also creates a potentially unstable anatomic situation. Adverse consequences include delayed union, malunion, and nonunion. Subacromial impingement, decreased strength and muscle fatigue, discomfort due to altered shoulder mechanics, neurovascular compromise due to a drooping shoulder, and glenohumeral degenerative joint disease also can occur. If unacceptable displacement is present, surgical reduction and stabilization at the injury sites is necessary. Frequently, operative management of 1 of the injury sites satisfactorily reduces and stabilizes the second disruption indirectly.12
Herscovici et al reported results in 9 patients with ipsilateral clavicular and glenoid neck fractures.13 Seven patients were treated surgically with plate fixation of the clavicular fracture and achieved excellent results. Two patients were treated without surgery and were found to have decreased range of motion (ROM), as well as drooping of the involved shoulder. The authors strongly recommended ORIF of the clavicle to prevent glenoid neck malunion.
Combined fractures of the distal clavicle and superior aspect of the glenoid cavity is another potentially unstable situation. Each disruption may lead to displacement at the other fracture site. If displacement of the clavicular fracture site is unacceptable, surgical reduction and stabilization is indicated, usually with a Kirschner-wire (K-wire) tension-band fixation construct. Because the proximal clavicular segment is attached to the superior glenoid-coracoid process fragment by means of the coracoclavicular ligaments, this may indirectly reduce and stabilize the glenoid cavity fracture satisfactorily. If not, the glenoid fracture may also require surgical management using the surgical techniques described.
Fixation of acromion fractures. (A) tension band construct; and (B) plate-screw fixation (most appropriate for proximal fractures).
Fracture of the coracoid or acromion process with a second disruption of the SSSC is another potentially unstable situation. If displacement at either or both sites is unacceptable, surgical management is indicated. For double disruptions consisting of both an acromion and a coracoid fracture, ORIF of the acromion may be all that is required (see Image above and Image 4 in Multimedia).
Relevant Anatomy
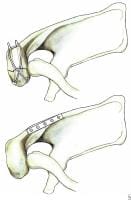
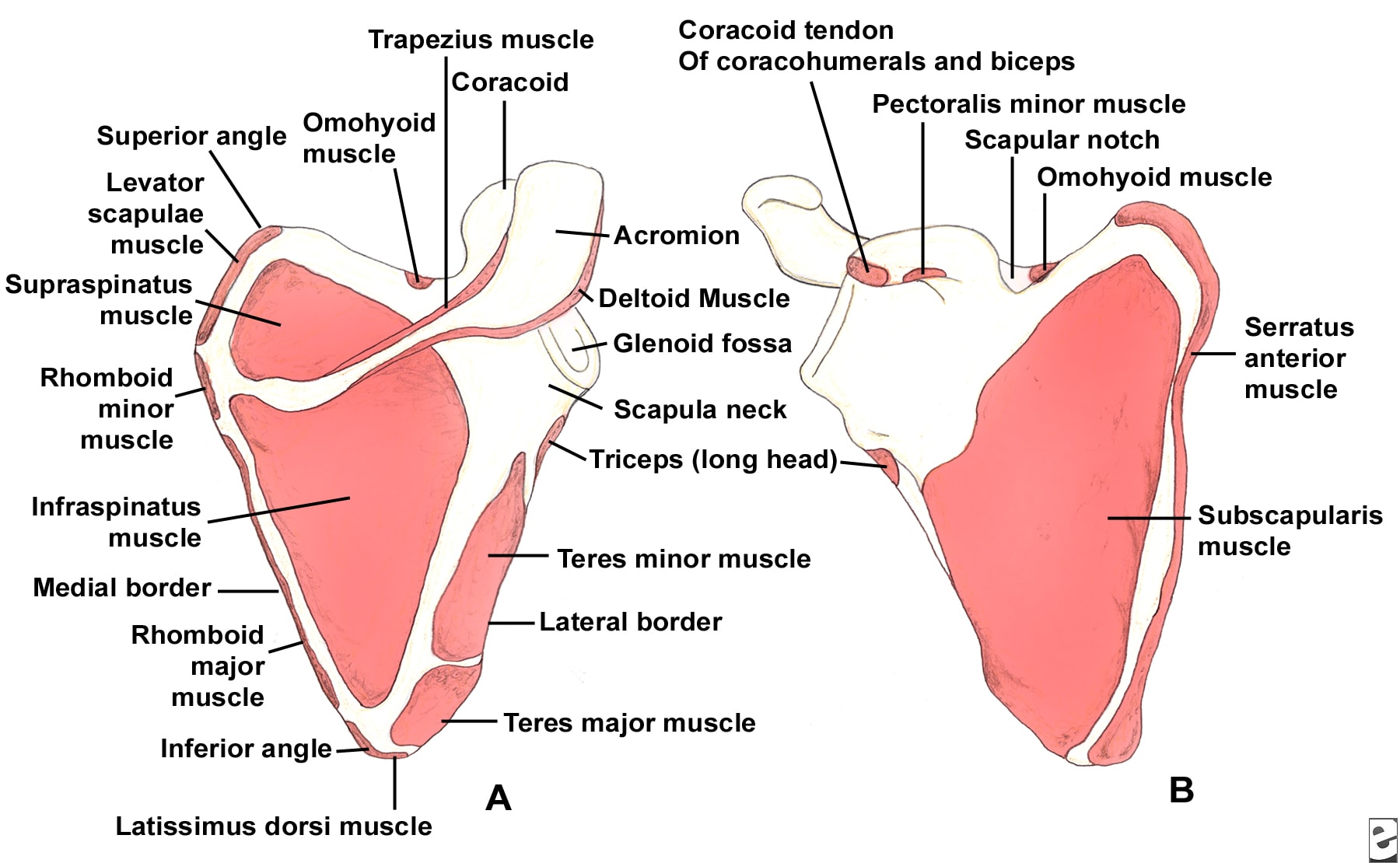
The scapula serves as the attachment site for 18 muscles, linking it to the thorax, spine, and upper extremity. The subscapularis muscle covers the anterior surface, and the serratus anterior muscle attaches to the inferior angle along the anterior medial border. The supraspinatus and infraspinatus muscles lie on the posterior border of the scapula. Overlying them is the trapezius, which inserts on the spine and the clavicle. The deltoid originates from the scapular spine, acromion, and anterior clavicle. Many other muscles attach to the scapular margins (see Image 5).
The coracoid process projects from the superior border of the scapula. The coracobrachialis and short head of the biceps muscles originate from the coracoid, and the pectoralis minor inserts on the coracoid. The brachial plexus and axillary artery run posterior to the pectoralis minor tendon. The scapular notch lies just medial to the coracoid base and is covered by the transverse scapular ligament. The suprascapular nerve runs under the ligament, and the suprascapular artery passes over it.
The acromion is the lateral projection from the spine of the scapula. The spinoglenoid notch is the gap between the acromion and the glenoid neck of the scapula. The suprascapular nerve and vessels pass through the notch en route to the infraspinatus.
Contraindications
Because scapula fractures often are associated with other, sometimes life-threatening injuries, delay surgery until the patient is medically stabilized. Absolute contraindications for surgery are few. In the case of a major vascular injury, such as an axillary or brachial artery tear, repair the vessel first, then follow with fracture fixation.
More on Scapula Fracture |
 Overview: Scapula Fracture Overview: Scapula Fracture |
| Workup: Scapula Fracture |
| Treatment: Scapula Fracture |
| Follow-up: Scapula Fracture |
| Multimedia: Scapula Fracture |



Tidak ada komentar:
Posting Komentar